riftpersiancat
Iron
- Joined
- Jan 28, 2020
- Posts
- 110
- Reputation
- 281
This post contains a list of the most viable and relevant hair regeneration treatments which are in development. They can be referred to as hair loss cures, baldness cures, hair growth treatments, hair loss treatments, or cures for alopecia. The treatments listed in this guide are given an estimated ranking based on their potential of hair growth efficacy and how soon the treatment can be released to the public market. #1 is the most relevant and so on.


1. Tissuse/J. Hewitt – TissUse’s hair multiplication technology “Smart Hair Transplant” or SHT has been licensed to the biopharmaceutical company J. Hewitt of Japan. Through this agreement J. Hewitt has rights to develop and commercialize the SHT procedure in Japan where regulations allow stem-cell based technologies to reach the market faster than anywhere else in the world. In September 2019, CEO of J. Hewitt, Jon Knight, told Follicle Thought that his company had ambitious plans to get SHT into a clinical trial quickly and “prove to the world” whether the treatment works. If successful, SHT would change the current paradigm of hair restoration. In the SHT procedure, 30 hair follicles are first extracted from the donor area of the scalp. Specific cells are isolated from the extracted follicles and then put through TissUse’s proprietary culturing technique. The company theorizes that from 30 hair follicles the SHT procedure can create 10,000 “neopapillae.” The neopapillae are hair promoting cells which are capable of growing new hair follicles and rejuvenating existing weakened hair.
Points of Interest: TissUse’s technology was developed by world-class hair follicle researchers Drs. Gerd Lindner and Roland Lauster. Their initial scientific paper on hair multiplication was published in 2011 and the technology has been continuously refined since then. With a clinical trial planned for January 2020, it is currently ahead of the pack of next-gen hair multiplication therapies.
Status: Preparing for a world’s first-in-human clinical trial for an advanced method of hair multiplication. The trial is planned to begin in 2020 in Japan according to the availability of a third party Cell Processing Center to conduct the trial.
2. Follica – Follica was founded in 2007 and its science is based on creating micro-wounds in the scalp to create hair follicle generation. Since the time of Follica’s original clinical trials (which did not bring about the desired results), Follica has continued its research and development on combining novel compounds with micro-wounding to proliferate hair follicle formation. The name of Follica’s treatment platform was originally called R.A.I.N. or regeneration abrasion induced neogenesis. In 2019 the treatment was officially titled FOL-004 in the company’s press releases. FOL-004 is a two part process which consists of 1) a micro wounding therapy combined with applied compounds which takes place in the clinic, 2) followed by a treatment package to be used at home consisting of a topical formula and an application device. Follica’s website, at one time, has also shown a smart phone app to help users keep track of their at-home treatment routines.
Points of Interest: Follica’s co-founder Dr. George Cotsarelis is one of the household names in the hair follicle research world and has an array of hair growth related patents under his belt. About 10 years deep in development, Follica is planning to initiate a pivotal trial in 2020, which, pending results, could lead to Follica’s hair growth therapy becoming market approved shortly thereafter. The in-office portion of the FOL-004 treatment is reported to take only 5 minutes.
Status: Preparing for FDA clearance trial in H1 2020.
 3. Shiseido/Replicel – Replicel’s hair growth treatment, RCH-01, involves culturing a person’s own hair follicle cells and then re-injecting them back into their scalp. First, a small punch-biopsy is removed from a person’s healthy hair follicles. Then, a specific cell is dissected from the follicle and cultured in a growth medium. The cells are replicated into the millions and then injected back into the person’s scalp. There is a short video about the procedure here. Replicel has been involved in a lot of activity over the past years to further develop their RCH-01 treatment into a worldwide success. For starters, Replicel created a partnership with Shiseido, which is the fourth largest cosmetic company in the world. In May 2014 Shiseido opened a huge biotechnology facility in Japan to accelerate the launch of Replicel’s hair technology RCH-01. This endeavor coincides perfectly with Japan’s new legislation which is designed to help expedite the trial process for stem cell technologies. In other words, Japan is an ideal place to launch a new cellular-based technology and get it to market quickly. To be clear, Shiseido and Replicel are separate companies. Replicel has licensed Shiseido the rights to bring RCH-01 to the world through an expedited regulatory path in Japan. It is worth mentioning that Shiseido also has its own scientists who are working on a separate hair regeneration technique using iPS cells. A treatment using iPS cells would be a further advancement and could potentially produce an unlimited amount of hair regrowth.
3. Shiseido/Replicel – Replicel’s hair growth treatment, RCH-01, involves culturing a person’s own hair follicle cells and then re-injecting them back into their scalp. First, a small punch-biopsy is removed from a person’s healthy hair follicles. Then, a specific cell is dissected from the follicle and cultured in a growth medium. The cells are replicated into the millions and then injected back into the person’s scalp. There is a short video about the procedure here. Replicel has been involved in a lot of activity over the past years to further develop their RCH-01 treatment into a worldwide success. For starters, Replicel created a partnership with Shiseido, which is the fourth largest cosmetic company in the world. In May 2014 Shiseido opened a huge biotechnology facility in Japan to accelerate the launch of Replicel’s hair technology RCH-01. This endeavor coincides perfectly with Japan’s new legislation which is designed to help expedite the trial process for stem cell technologies. In other words, Japan is an ideal place to launch a new cellular-based technology and get it to market quickly. To be clear, Shiseido and Replicel are separate companies. Replicel has licensed Shiseido the rights to bring RCH-01 to the world through an expedited regulatory path in Japan. It is worth mentioning that Shiseido also has its own scientists who are working on a separate hair regeneration technique using iPS cells. A treatment using iPS cells would be a further advancement and could potentially produce an unlimited amount of hair regrowth.
Points of Interest: Shiseido trialed Replicel’s RCH-01 in Japan from mid 2016 – 2018. Since then, no results from the trial have been shared by the company which has brought about speculation on its outcome. Replicel has announced in 2019 that they are actively engaging with Shiseido to get the trial results released. By the end of 2020 we should know whether this treatment will be useful to hair loss patients worldwide or not.
Status: Awaiting disclosure of Shiseido’s trial results from RCH-01 which will determine the future of the product.
 4. Samumed – SM04554 is a small-molecule topical solution that activates the Wnt pathway to grow hair. In March 2016 Samumed presented their highly anticipated phase 2 results at the American Academy of Dermatology. The Phase 2 results got a mixed response from the online world of hair growth enthusiasts, but ultimately, the treatment did grow hair in the trial. The best responding group from the trial showed an approximate 10% overall increase in hair density. This phase 2 trial also lead to the conclusion that SM04554 does not create a “dose response” in patients who use it. This means that adding more of the drug to a patient’s biological system does not lead to increased results. Finding the “just right” dosage and application is important for this drug to work its best.
4. Samumed – SM04554 is a small-molecule topical solution that activates the Wnt pathway to grow hair. In March 2016 Samumed presented their highly anticipated phase 2 results at the American Academy of Dermatology. The Phase 2 results got a mixed response from the online world of hair growth enthusiasts, but ultimately, the treatment did grow hair in the trial. The best responding group from the trial showed an approximate 10% overall increase in hair density. This phase 2 trial also lead to the conclusion that SM04554 does not create a “dose response” in patients who use it. This means that adding more of the drug to a patient’s biological system does not lead to increased results. Finding the “just right” dosage and application is important for this drug to work its best.
Points of Interest: Samumed began by breezing their way through clinical trials and completed an important phase 2 within about two years time from their initial startup. The company has even reached the phase 3 level for its drug which is rare in the alopecia field.The phase 3 trial for SM04554 began in November 2018 and is scheduled to be completed in January 2021.
Status: Samumed is currently undergoing a Phase 3 trial for SM04554 in Turkey. If the trial is successful, the product could become commercially available in Turkey in late 2021.

5. Cassiopea – Breezula is the brand name of the topical anti-androgen developed by Cassiopea for the treatment of AGA. The actual drug itself is called clascoterone and is also used to treat acne. Unlike other anti-androgens we have seen which block the body’s production of DHT, Breezula instead works by blocking DHT’s ability to bind to androgen receptors in the hair follicle. It is also claimed that Breezula reduces the skin’s production of prostaglandin D2 (PGD2), a biological compound which, in elevated levels, has been shown to inhibit hair growth. Cortexolone-17α-propionate, the active ingredient in Breezula, is also being trialed by Cassiopea to treat acne. In July 2018, Cassiopea issued a press release describing positive data midway through the phase 2 dose ranging trial. In April 2019 another press release was issued detailing the fully completed 12 month trial.
Points of Interest: Clascoterone/Breezula is a topical anti-androgen with (reportedly) no hormonal side effects. The company states that the drug does not interfere with the androgenic profile of treated patients. Cassiopea has stated the early phase 2 results Breezula produced at 6 months were comparable to oral Propecia’s results in a 12 month trial.
Status: Has completed a Phase 2 dose ranging study with positive data announced in April 2019. Cassiopea plans to begin a Phase 3 clinical trial in H1 2020. Expected market launch in 2022.

6. Follicum – Follicum is a Swedish biotechnology company that is developing human peptides for the use of stimulating hair growth, and potentially inhibiting hair growth. The lead candidate peptide of Follicum’s pipeline is called FOL-005. In its first clinical trial the peptide was tested on the thighs of human patients and displayed an increase in hair growth in the treated areas. FOL-005 was subsequently tested in a phase 2 trial on human scalps by injection delivery. That trial showed FOL-005 was able to create an average increase of 7 hair per cm² among the trial subjects. Follicum has since developed a topical cream-like version of their peptide to make the treatment more user-friendly. In 2020, a topical version of FOL-005 will be tested on human scalps and will be a decisive moment for the potential hair growth drug.
Points of Interest: Follicum debuted a graph of FOL-005’s pre-clinical trial results on Follicle Thought, which were impressive. Follicum has been working with renowned hair researcher Ralf Paus since 2012 to further their R&D. In late 2019, Follicum announced that a topical formulation of their peptide has been completed through collaboration with Bioglan and is ready to be trialed.
Status: A Phase 2B trial using a topical version of FOL-005 is expected to begin around January 2020.

7. HairClone – HairClone is a British biotechnology company seeking to rejuvenate hair through the use of cultured dermal papilla cells. The company has put together an interesting business model while also leveraging scientific innovations and a advantageous regulatory pathway. HairClone envisions patients banking a few of their hair follicles in a deep freeze storage facility and then utilizing those follicles periodically over the course of years to create cellular cocktails to be injected back into their scalp. This is an innovative approach to cellular hair growth therapy and makes the process of undergoing repeat procedures easier for patients. HairClone anticipates that their cellular therapy will rejuvenate existing hair follicles that have become weakened. The company also anticipates that cellular injections could convert DHT-susceptible follicles into DHT-resistant follicles. Key leadership for the company includes Paul Kemp PhD, Vincent Ranford PhD, and well known hair surgeons Drs. Bessam and Nilofer Farjo. A news video describing the HairClone procedure was reported in 2017.
Points of Interest: The founders of HairClone were responsible for the first company to attempt hair regeneration via cell therapy (Intercytex) in the late 2000’s. HairClone has assembled a robust scientific advisory board including well known researcher Dr. Claire Higgins. The procedure is projected to involve only one surgery to extract 50 hair follicles via FUE, place those follicles into cryopreservation, and use them as needed over the following years. HairClone estimates a patient may want to receive follow-up injections every 2-3 years to maintain optimum hair health. The company unveiled a crowdfunding campaign in 2018 which did not meet its goal. After the failed attempt, HairClone is left seeking alternate means to raise capital for its development.
Status: Currently fundraising for a GMP licensed facility to create expanded dermal papilla cell cultures in. HairClone expects to offer expanded cell treatments through practitioners in the UK in 2020/21.
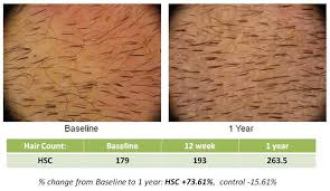
8. Histogen – Histogen’s Hair Stimulating Complex (HST-01) is a cell conditioned media that is derived from neonatal cells grown under embryonic-like conditions. It is an injectable serum that is used to stimulate the growth of new hair follicles as well as existing ones in a person’s scalp. This treatment was first announced around 2008 and a lot of people are eager for it to be released on the market. HSC has shown visual evidence of its efficacy and has had great numbers in clinical trials. The treatment has been trialed on both men and women and is said to be effective at regrowing hair at the temples as well as throughout the scalp. Compared to its initial inception, the current HSC product has been refined over the past few years to contain less cellular impurities and be a more concentrated formula of growth factors. After a long drought of news or progress, Histogen carried out a phase 1 female trial in the US in 2018.
Points of Interest: This is a treatment that has a very high appeal due to its ease of application. Theoretically, you could walk in, receive injections, and walk out. No need to harvest your own cells and come back to get them re-injected such as with other cellular based treatments. If a larger pharmaceutical company (such as Allergan) would be interested in partnering with Histogen, the HST-01 product would likely speed through clinical trials and become a highly successful product. It could be implemented in offices that offer aesthetic injectables such as botox or collagen.
Status: Phase 1 trial for female alopecia was successfully completed in July 2019. A Phase 1B for men is anticipated to begin in early 2020.

9. RiverTown Therapeutics Inc. – RT1640 is composed of minoxidil, cyclosporine-A, and a novel molecule RT175. This compound was developed by the founder of RiverTown Therapeutics Inc., David Weinstein MD, PhD. In a pilot trial, 100% of the people who used the treatment had satisfactory hair growth and, reportedly, several people had significant hair regeneration. RiverTown Therapeutics debuted their fantastic hair growth results on Follicle Thought. The three agents in RT1640 act on distinct pathways of AGA and synergize to promote the growth and maintenance of hair follicles. RT1640 also reanimates the melanocytic progenitor cells which give hair its color, hence, RT1640 is said to restore pigment to some of the regenerated hair as well. Interestingly enough, RT1640 was brought to life through its founder’s personal interest in treating his own baldness. RiverTown was featured in a popular article for the New Yorker magazine in June 2018.
Points of Interest: The results for RT1640 are promising for a topical treatment. Not only does RT1640 regrow hair, it is also able to restore pigment to some of the hair it regenerates. The three agents that compose RT1640 have strong safety profiles and have all been tested in clinical trials previously.
Status: Currently seeking investment or strategic partners in order to initiate a Phase 1B/2A study in 2020.

10. Tsuji-Riken/Organ Technologies – Dr. Takashi Tsuji runs one of the most advanced stem cell labs in the world at the Riken Institute in Japan. In mid-2016 it was announced that Riken would be establishing a joint venture with the electronics company Kyocera and the regenerative medicine company Organ Technologies to bring Dr. Tsuji’s hair regeneration treatment to the market. In the joint venture Kyocera will be developing the cell processing devices and RIKEN and Organ Technologies will be responsible for the stem cell culturing and manipulation, the production process, and implementation of the preclinical trials. The treatment involves extracting a small number of hair follicles from a person’s donor scalp area and then isolating two specific types of cells from the follicle – papilla cells and epidermal cells from the bulge region. These cells are then cultured, expanded, and combined to create a hair follicle “germ” or “follicular primordium.” Once the hair follicle germs are ready they are transported to a facility where they can be implanted back into a person’s scalp to grow hair. Many people have speculated that this type of hair cloning technology could be a holy grail baldness cure.
Points of Interest: Tsuji has been doing hair follicle research for several years now and it’s awesome that the strategic partners are finally in place to bring his hair growth treatment to the world. Organ Technologies will develop this treatment in Japan which currently has the fastest track to market approval in the world for cellular therapies.In June 2018, RIKEN announced their lab is moving forward with animal studies for a newly refined protocol, and if successful they would subsequently initiate human trials.
Status: Currently undergoing research and development with a goal to begin clinical trials in 2020 if preclinical research goes well.

11. Stemson Therapeutics – Stemson Therapeutics is a hair multiplication company based in La Jolla, CA. The company has been founded by Dr. Alexey Terskikh who is known from his research on hair cloning at the Sandford Burnham Prebys Institute. The first iteration of Terskikh’s research in hair cloning made headlines in 2015 when he was able to grow hair on mice using human induced pluripotent stem cells which were converted into dermal papilla cells. In 2019, the Sanford Burnham Prebys Institute announced that Terskikh had created a separate company (Stemson) to further develop his hair cloning research into a commercial therapy. The company now stands as North America’s front runner in the hair cloning industry. CEO Geoff Hamilton has told the media that Stemson is hoping to begin human trials around the beginning of 2021.
Points of Interest: Stemson claims that they only need a small tissue sample from a patient, even blood, to create an unlimited supply of hair producing “folliculogenic” cells. In June 2019, Stemson announced it had received a $3 million investment from the pharmaceutical powerhouse Allergan. Stemson also claims that it will be possible in the future to create allogeneic treatments with their technology. This means a patient will not need to harvest their own cells to produce the folliculogenic cells and could simply receive injections from a compatible cell line developed by Stemson.
Status: Currently developing a hair multiplication therapy using all human cells and a biodegradable scaffold which guides the direction of hair growth through the skin. Aiming at human trials in late 2020/H1 2021.

12. Stemore – Stemore is a biotechnology company from South Korea that is a developing a wide range of therapies for hair loss. The company was founded by Dr. Sung Jong Hyuk of Yonsei University. Stemore’s primary target is creating an effective dermal papilla cell therapy. Dr. Sung and his team have completed numerous studies on dermal papilla enhancement and adipose-derived stem cell enhancement. In late 2019, Stemore completed an exclusive interview with Follicle Thought which explained the company’s therapies in great detail. Depending on how development progresses for Stemore in 2020, they may end up becoming a major player in this industry within a short amount of time.
Points of Interest: Stemore has multiple cell therapies in their pipeline as well as topical cosmetic products for hair growth. The company’s technology specializes in maintaining the hair growth capacity of dermal papilla cells, even after being cultured multiple times.
Status: Preparing for clinical trials for a dermal papilla cell injection therapy and an adipose-derived stem cell injection therapy in 2020. Cosmetic products SM-P2 and Marliolide are scheduled to be released in 2020.

13. Rapunzel – Two of the most prominent names when it comes to hair follicle research, Dr. Colin Jahoda and Dr. Angela Christiano, have teamed up to bring an effective hair loss cure to the masses. Dr. Jahoda’s latest public work has been focused on 3D dermal papilla culturing, while Dr. Christiano has been popping up frequently in news headlines for her research on JAK inhibitors and hair growth. Dr. Christiano has even recently sold her JAK IP to Aclaris Therapeutics who is carrying out trials for JAK inhibtor’s use in treating AGA and alopecia areata. Even better, these two researchers are reportedly teaming up on Christiano’s new startup “Rapunzel” to develop a treatment using cultured hair follicle cells to regenerate hair. Christiano has recently stated “we can grow rat hair like it’s no tomorrow, but we think we can do it with human hair, too.”
Points of Interest: Dr. Jahoda implanted his own hair cells into his wife’s arm and found that his cells grew hair on her arm all the way back in 1999. In other words, the man is experienced in cellular hair growth research. Also, Jahoda and Christiano have both been issued a new patent related to 3D hair follicle culturing in January 2017.
Status: Undergoing preclinical work.
1. Tissuse/J. Hewitt – TissUse’s hair multiplication technology “Smart Hair Transplant” or SHT has been licensed to the biopharmaceutical company J. Hewitt of Japan. Through this agreement J. Hewitt has rights to develop and commercialize the SHT procedure in Japan where regulations allow stem-cell based technologies to reach the market faster than anywhere else in the world. In September 2019, CEO of J. Hewitt, Jon Knight, told Follicle Thought that his company had ambitious plans to get SHT into a clinical trial quickly and “prove to the world” whether the treatment works. If successful, SHT would change the current paradigm of hair restoration. In the SHT procedure, 30 hair follicles are first extracted from the donor area of the scalp. Specific cells are isolated from the extracted follicles and then put through TissUse’s proprietary culturing technique. The company theorizes that from 30 hair follicles the SHT procedure can create 10,000 “neopapillae.” The neopapillae are hair promoting cells which are capable of growing new hair follicles and rejuvenating existing weakened hair.
Points of Interest: TissUse’s technology was developed by world-class hair follicle researchers Drs. Gerd Lindner and Roland Lauster. Their initial scientific paper on hair multiplication was published in 2011 and the technology has been continuously refined since then. With a clinical trial planned for January 2020, it is currently ahead of the pack of next-gen hair multiplication therapies.
Status: Preparing for a world’s first-in-human clinical trial for an advanced method of hair multiplication. The trial is planned to begin in 2020 in Japan according to the availability of a third party Cell Processing Center to conduct the trial.
2. Follica – Follica was founded in 2007 and its science is based on creating micro-wounds in the scalp to create hair follicle generation. Since the time of Follica’s original clinical trials (which did not bring about the desired results), Follica has continued its research and development on combining novel compounds with micro-wounding to proliferate hair follicle formation. The name of Follica’s treatment platform was originally called R.A.I.N. or regeneration abrasion induced neogenesis. In 2019 the treatment was officially titled FOL-004 in the company’s press releases. FOL-004 is a two part process which consists of 1) a micro wounding therapy combined with applied compounds which takes place in the clinic, 2) followed by a treatment package to be used at home consisting of a topical formula and an application device. Follica’s website, at one time, has also shown a smart phone app to help users keep track of their at-home treatment routines.
Points of Interest: Follica’s co-founder Dr. George Cotsarelis is one of the household names in the hair follicle research world and has an array of hair growth related patents under his belt. About 10 years deep in development, Follica is planning to initiate a pivotal trial in 2020, which, pending results, could lead to Follica’s hair growth therapy becoming market approved shortly thereafter. The in-office portion of the FOL-004 treatment is reported to take only 5 minutes.
Status: Preparing for FDA clearance trial in H1 2020.

Points of Interest: Shiseido trialed Replicel’s RCH-01 in Japan from mid 2016 – 2018. Since then, no results from the trial have been shared by the company which has brought about speculation on its outcome. Replicel has announced in 2019 that they are actively engaging with Shiseido to get the trial results released. By the end of 2020 we should know whether this treatment will be useful to hair loss patients worldwide or not.
Status: Awaiting disclosure of Shiseido’s trial results from RCH-01 which will determine the future of the product.

Points of Interest: Samumed began by breezing their way through clinical trials and completed an important phase 2 within about two years time from their initial startup. The company has even reached the phase 3 level for its drug which is rare in the alopecia field.The phase 3 trial for SM04554 began in November 2018 and is scheduled to be completed in January 2021.
Status: Samumed is currently undergoing a Phase 3 trial for SM04554 in Turkey. If the trial is successful, the product could become commercially available in Turkey in late 2021.

5. Cassiopea – Breezula is the brand name of the topical anti-androgen developed by Cassiopea for the treatment of AGA. The actual drug itself is called clascoterone and is also used to treat acne. Unlike other anti-androgens we have seen which block the body’s production of DHT, Breezula instead works by blocking DHT’s ability to bind to androgen receptors in the hair follicle. It is also claimed that Breezula reduces the skin’s production of prostaglandin D2 (PGD2), a biological compound which, in elevated levels, has been shown to inhibit hair growth. Cortexolone-17α-propionate, the active ingredient in Breezula, is also being trialed by Cassiopea to treat acne. In July 2018, Cassiopea issued a press release describing positive data midway through the phase 2 dose ranging trial. In April 2019 another press release was issued detailing the fully completed 12 month trial.
Points of Interest: Clascoterone/Breezula is a topical anti-androgen with (reportedly) no hormonal side effects. The company states that the drug does not interfere with the androgenic profile of treated patients. Cassiopea has stated the early phase 2 results Breezula produced at 6 months were comparable to oral Propecia’s results in a 12 month trial.
Status: Has completed a Phase 2 dose ranging study with positive data announced in April 2019. Cassiopea plans to begin a Phase 3 clinical trial in H1 2020. Expected market launch in 2022.

6. Follicum – Follicum is a Swedish biotechnology company that is developing human peptides for the use of stimulating hair growth, and potentially inhibiting hair growth. The lead candidate peptide of Follicum’s pipeline is called FOL-005. In its first clinical trial the peptide was tested on the thighs of human patients and displayed an increase in hair growth in the treated areas. FOL-005 was subsequently tested in a phase 2 trial on human scalps by injection delivery. That trial showed FOL-005 was able to create an average increase of 7 hair per cm² among the trial subjects. Follicum has since developed a topical cream-like version of their peptide to make the treatment more user-friendly. In 2020, a topical version of FOL-005 will be tested on human scalps and will be a decisive moment for the potential hair growth drug.
Points of Interest: Follicum debuted a graph of FOL-005’s pre-clinical trial results on Follicle Thought, which were impressive. Follicum has been working with renowned hair researcher Ralf Paus since 2012 to further their R&D. In late 2019, Follicum announced that a topical formulation of their peptide has been completed through collaboration with Bioglan and is ready to be trialed.
Status: A Phase 2B trial using a topical version of FOL-005 is expected to begin around January 2020.

7. HairClone – HairClone is a British biotechnology company seeking to rejuvenate hair through the use of cultured dermal papilla cells. The company has put together an interesting business model while also leveraging scientific innovations and a advantageous regulatory pathway. HairClone envisions patients banking a few of their hair follicles in a deep freeze storage facility and then utilizing those follicles periodically over the course of years to create cellular cocktails to be injected back into their scalp. This is an innovative approach to cellular hair growth therapy and makes the process of undergoing repeat procedures easier for patients. HairClone anticipates that their cellular therapy will rejuvenate existing hair follicles that have become weakened. The company also anticipates that cellular injections could convert DHT-susceptible follicles into DHT-resistant follicles. Key leadership for the company includes Paul Kemp PhD, Vincent Ranford PhD, and well known hair surgeons Drs. Bessam and Nilofer Farjo. A news video describing the HairClone procedure was reported in 2017.
Points of Interest: The founders of HairClone were responsible for the first company to attempt hair regeneration via cell therapy (Intercytex) in the late 2000’s. HairClone has assembled a robust scientific advisory board including well known researcher Dr. Claire Higgins. The procedure is projected to involve only one surgery to extract 50 hair follicles via FUE, place those follicles into cryopreservation, and use them as needed over the following years. HairClone estimates a patient may want to receive follow-up injections every 2-3 years to maintain optimum hair health. The company unveiled a crowdfunding campaign in 2018 which did not meet its goal. After the failed attempt, HairClone is left seeking alternate means to raise capital for its development.
Status: Currently fundraising for a GMP licensed facility to create expanded dermal papilla cell cultures in. HairClone expects to offer expanded cell treatments through practitioners in the UK in 2020/21.

8. Histogen – Histogen’s Hair Stimulating Complex (HST-01) is a cell conditioned media that is derived from neonatal cells grown under embryonic-like conditions. It is an injectable serum that is used to stimulate the growth of new hair follicles as well as existing ones in a person’s scalp. This treatment was first announced around 2008 and a lot of people are eager for it to be released on the market. HSC has shown visual evidence of its efficacy and has had great numbers in clinical trials. The treatment has been trialed on both men and women and is said to be effective at regrowing hair at the temples as well as throughout the scalp. Compared to its initial inception, the current HSC product has been refined over the past few years to contain less cellular impurities and be a more concentrated formula of growth factors. After a long drought of news or progress, Histogen carried out a phase 1 female trial in the US in 2018.
Points of Interest: This is a treatment that has a very high appeal due to its ease of application. Theoretically, you could walk in, receive injections, and walk out. No need to harvest your own cells and come back to get them re-injected such as with other cellular based treatments. If a larger pharmaceutical company (such as Allergan) would be interested in partnering with Histogen, the HST-01 product would likely speed through clinical trials and become a highly successful product. It could be implemented in offices that offer aesthetic injectables such as botox or collagen.
Status: Phase 1 trial for female alopecia was successfully completed in July 2019. A Phase 1B for men is anticipated to begin in early 2020.

9. RiverTown Therapeutics Inc. – RT1640 is composed of minoxidil, cyclosporine-A, and a novel molecule RT175. This compound was developed by the founder of RiverTown Therapeutics Inc., David Weinstein MD, PhD. In a pilot trial, 100% of the people who used the treatment had satisfactory hair growth and, reportedly, several people had significant hair regeneration. RiverTown Therapeutics debuted their fantastic hair growth results on Follicle Thought. The three agents in RT1640 act on distinct pathways of AGA and synergize to promote the growth and maintenance of hair follicles. RT1640 also reanimates the melanocytic progenitor cells which give hair its color, hence, RT1640 is said to restore pigment to some of the regenerated hair as well. Interestingly enough, RT1640 was brought to life through its founder’s personal interest in treating his own baldness. RiverTown was featured in a popular article for the New Yorker magazine in June 2018.
Points of Interest: The results for RT1640 are promising for a topical treatment. Not only does RT1640 regrow hair, it is also able to restore pigment to some of the hair it regenerates. The three agents that compose RT1640 have strong safety profiles and have all been tested in clinical trials previously.
Status: Currently seeking investment or strategic partners in order to initiate a Phase 1B/2A study in 2020.

10. Tsuji-Riken/Organ Technologies – Dr. Takashi Tsuji runs one of the most advanced stem cell labs in the world at the Riken Institute in Japan. In mid-2016 it was announced that Riken would be establishing a joint venture with the electronics company Kyocera and the regenerative medicine company Organ Technologies to bring Dr. Tsuji’s hair regeneration treatment to the market. In the joint venture Kyocera will be developing the cell processing devices and RIKEN and Organ Technologies will be responsible for the stem cell culturing and manipulation, the production process, and implementation of the preclinical trials. The treatment involves extracting a small number of hair follicles from a person’s donor scalp area and then isolating two specific types of cells from the follicle – papilla cells and epidermal cells from the bulge region. These cells are then cultured, expanded, and combined to create a hair follicle “germ” or “follicular primordium.” Once the hair follicle germs are ready they are transported to a facility where they can be implanted back into a person’s scalp to grow hair. Many people have speculated that this type of hair cloning technology could be a holy grail baldness cure.
Points of Interest: Tsuji has been doing hair follicle research for several years now and it’s awesome that the strategic partners are finally in place to bring his hair growth treatment to the world. Organ Technologies will develop this treatment in Japan which currently has the fastest track to market approval in the world for cellular therapies.In June 2018, RIKEN announced their lab is moving forward with animal studies for a newly refined protocol, and if successful they would subsequently initiate human trials.
Status: Currently undergoing research and development with a goal to begin clinical trials in 2020 if preclinical research goes well.

11. Stemson Therapeutics – Stemson Therapeutics is a hair multiplication company based in La Jolla, CA. The company has been founded by Dr. Alexey Terskikh who is known from his research on hair cloning at the Sandford Burnham Prebys Institute. The first iteration of Terskikh’s research in hair cloning made headlines in 2015 when he was able to grow hair on mice using human induced pluripotent stem cells which were converted into dermal papilla cells. In 2019, the Sanford Burnham Prebys Institute announced that Terskikh had created a separate company (Stemson) to further develop his hair cloning research into a commercial therapy. The company now stands as North America’s front runner in the hair cloning industry. CEO Geoff Hamilton has told the media that Stemson is hoping to begin human trials around the beginning of 2021.
Points of Interest: Stemson claims that they only need a small tissue sample from a patient, even blood, to create an unlimited supply of hair producing “folliculogenic” cells. In June 2019, Stemson announced it had received a $3 million investment from the pharmaceutical powerhouse Allergan. Stemson also claims that it will be possible in the future to create allogeneic treatments with their technology. This means a patient will not need to harvest their own cells to produce the folliculogenic cells and could simply receive injections from a compatible cell line developed by Stemson.
Status: Currently developing a hair multiplication therapy using all human cells and a biodegradable scaffold which guides the direction of hair growth through the skin. Aiming at human trials in late 2020/H1 2021.

12. Stemore – Stemore is a biotechnology company from South Korea that is a developing a wide range of therapies for hair loss. The company was founded by Dr. Sung Jong Hyuk of Yonsei University. Stemore’s primary target is creating an effective dermal papilla cell therapy. Dr. Sung and his team have completed numerous studies on dermal papilla enhancement and adipose-derived stem cell enhancement. In late 2019, Stemore completed an exclusive interview with Follicle Thought which explained the company’s therapies in great detail. Depending on how development progresses for Stemore in 2020, they may end up becoming a major player in this industry within a short amount of time.
Points of Interest: Stemore has multiple cell therapies in their pipeline as well as topical cosmetic products for hair growth. The company’s technology specializes in maintaining the hair growth capacity of dermal papilla cells, even after being cultured multiple times.
Status: Preparing for clinical trials for a dermal papilla cell injection therapy and an adipose-derived stem cell injection therapy in 2020. Cosmetic products SM-P2 and Marliolide are scheduled to be released in 2020.

13. Rapunzel – Two of the most prominent names when it comes to hair follicle research, Dr. Colin Jahoda and Dr. Angela Christiano, have teamed up to bring an effective hair loss cure to the masses. Dr. Jahoda’s latest public work has been focused on 3D dermal papilla culturing, while Dr. Christiano has been popping up frequently in news headlines for her research on JAK inhibitors and hair growth. Dr. Christiano has even recently sold her JAK IP to Aclaris Therapeutics who is carrying out trials for JAK inhibtor’s use in treating AGA and alopecia areata. Even better, these two researchers are reportedly teaming up on Christiano’s new startup “Rapunzel” to develop a treatment using cultured hair follicle cells to regenerate hair. Christiano has recently stated “we can grow rat hair like it’s no tomorrow, but we think we can do it with human hair, too.”
Points of Interest: Dr. Jahoda implanted his own hair cells into his wife’s arm and found that his cells grew hair on her arm all the way back in 1999. In other words, the man is experienced in cellular hair growth research. Also, Jahoda and Christiano have both been issued a new patent related to 3D hair follicle culturing in January 2017.
Status: Undergoing preclinical work.